
The common explanation for this phenomenon is that proteins fold in order to reach the minimal level of energy. Different AA have different chemical, electrical, and size properties, and therefore two different folds of a protein usually have two different levels of energy.
We will use Van der Waals radius balls as a 3D model of an atom.
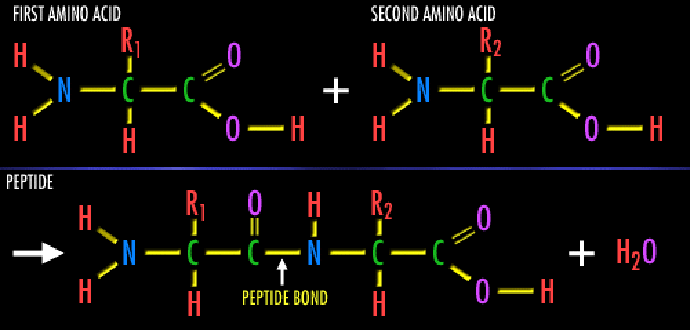
Each AA has a carbon atom called ![]() ,
connected to a carboxyl group
and an amine group, a hydrogen atom and a part that depends on the specific AA -
the residue.
Amine group of one AA connects to the carboxyl group of the next adjacent
AA (see figure 13.2). The
,
connected to a carboxyl group
and an amine group, a hydrogen atom and a part that depends on the specific AA -
the residue.
Amine group of one AA connects to the carboxyl group of the next adjacent
AA (see figure 13.2). The ![]() -s form together
a backbone wire, to which the rest of the atoms are attached.
-s form together
a backbone wire, to which the rest of the atoms are attached.
 |