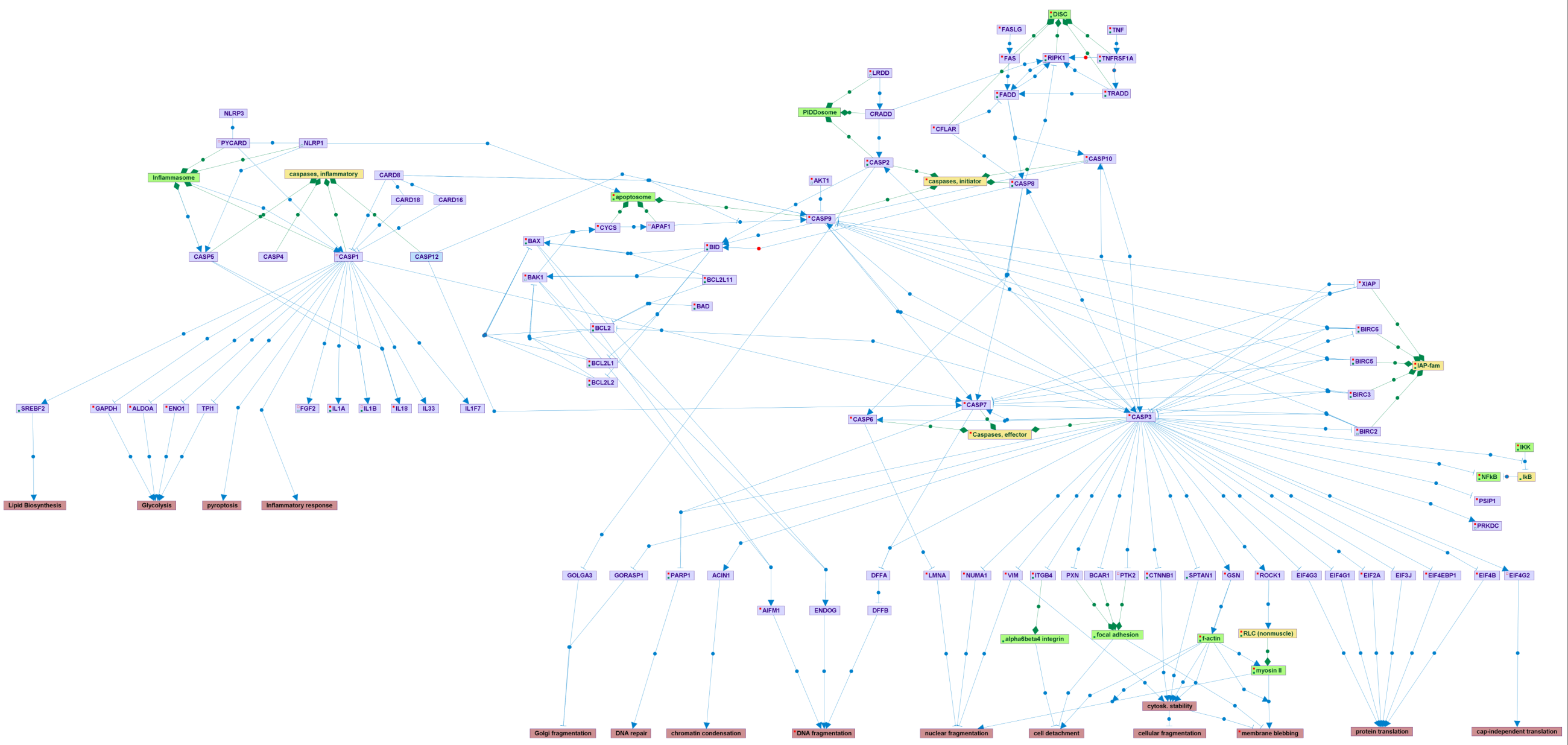
Caspases, the family of cysteine proteases whose proteolytic activity is the main mechanism by which apoptosis is executed, serve the core of a wide network of proteins that include activators, regulators, and substrates. Following death signals, caspases can be activated by the intrinsic mitochondrial-based pathway, in which internal death cues induce the formation of the apoptosome, a platform for activation of the apical initiator caspase-9, or by the extrinsic pathway, in which activation of death receptors by their extracellular ligands leads to DISC formation and activation of caspase-8 or 10. An additional mechanism for activating caspases involves the Piddosome, by which caspase-2 is activated. These inititator caspases in turn trigger a proteolyctic casacade leading to the activation of the effector caspases, especially caspase-3, which cleave numerous cellular substrates. This map details the physiologically relevant caspase substrates, and the particular cellular phenotypes that are induced by their cleavage. Several regulators modulate the activation steps, and caspases are directly inhibited by the IAP family of proteins. In addition to the initiator and effector caspases, a third sub-family exists, consisting of caspase-1 and its close relatives, which upon activation by the inflammasome, cleave various substrates, including cytoskines, that are involved in inflammatory responses.