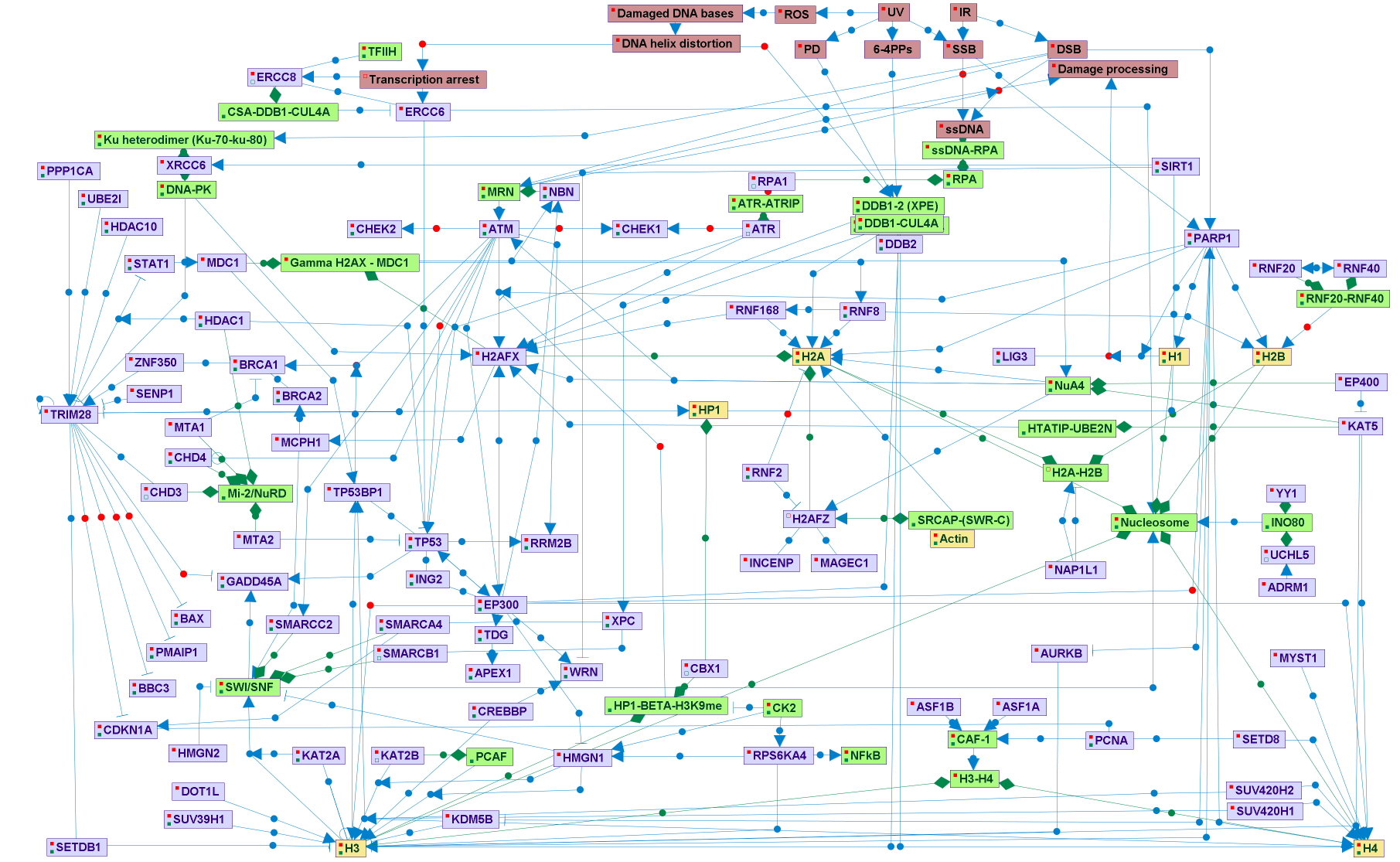
DNA damages, including single- and double- strand breaks (SSB and DSB respectively), pyrimidine dimmers (PD), and oxidized nucleotides, are caused by physical agents in the environment, like ionizing radiation (IR) and ultra violet light (UV), and by various chemicals (including reactive oxygen species (ROS) from internal metabolic processes source). In eukaryotes, the sensing and repair of DNA damages occur in chromatin context. Chromatin constitutes a physical barrier to enzymes and regulatory factors to reach the DNA. To deal with this obstacle, transient chromatin structural changes are integral to base- and nucleotide-excision, homologous recombination, and non-homologous end joining DNA repair pathways, Chromatin remodeling mechanisms includes: (1) posttranslational modifications (PTMs) of histones and non-histones chromatin associated proteins, including covalent additions (removals) of phosphoryl-, methyl-, acetyl-, ubiquitin-, SUMO, and ADP-ribose (2) histone-variants replacement, and nucleosomes repositioning or eviction. ATP-dependent multi subunits chromatin remodeling complexes (INO80, SRCAP/SWR-C, SWI/SNF, and MI-2/NuRD.) implement the latter mechanisms. PARP1 and the DDB1-2-CUL4A complex are early sensors of SSB, DSB, and PD DNA lesions. These proteins have chromatin-remodeling enzymatic activity. Additional early DSB sensors are the MRN and Ku70-Ku80 complexes, that activate ATM and DNA-PKcs kinases respectively, both belonging to the phosphoinositide-3-kinase-related protein kinases (PIKK). Upon SSB repair pathways, ATR, a third member of the PIKK kinases is activated. The PIKK kinases phosphorylate many substrates, including histones and proteins involved in regulation of chromatin structure. One of these proteins is TRIM28 (KAP1), a transcription co-repressor proposed to regulate chromatin structure and heterochromatin formation. In response to the induction of DNA DSBs, its co-repression function is inhibited byATM-dependant phospohorylation. The main histone PTMs in response to DSBs are those related to H2AFX (histone H2A variant). DDR-related replacement of H2A variant in the nucleosomes by H2AFZ (promoted by the SRCAP/SWR-C ATP-dependent complex) also occured. Upon DSB, the histone acetylase complex NUA4/TIP60, several histones ubiquitin ligases are activated. The interactions between the DDR proteins, MCPH1, P53 and BRCA1, and components of the SWI/SNF and MI-2/NuRD chromatin remodeling complexes, seems to be important to the regulation of DDR.